5.3: Exponential and Logarithmic Models
( \newcommand{\kernel}{\mathrm{null}\,}\)
While we have explored some basic applications of exponential and logarithmic functions, in this section we explore some important applications in more depth.
More complex exponential equations can often be solved in more than one way. In the following example, we will solve the same problem in two ways – one using logarithm properties, and the other using exponential properties.
Example 5.3.1a
In 2008, the population of Kenya was approximately 38.8 million, and was growing by 2.64% each year, while the population of Sudan was approximately 41.3 million and growing by 2.24% each year[1]. If these trends continue, when will the population of Kenya match that of Sudan?
[1] World Bank, World Development Indicators, as reported on http://www.google.com/publicdata, retrieved August 24, 2010
Solution
We start by writing an equation for each population in terms of t, the number of years after 2008.
Kenya(t)=38.8(1+0.0264)tSudan(t)=41.3(1+0.0224)t
To find when the populations will be equal, we can set the equations equal
38.8(1.0264)t=41.3(1.0224)t
For our first approach, we take the log of both sides of the equation
log(38.8(1.0264)t)=log(41.3(1.0224)t)
Utilizing the sum property of logs, we can rewrite each side,
log(38.8)+log(1.0264t)=log(41.3)+log(1.0224t)
Then utilizing the exponent property, we can pull the variables out of the exponent
log(38.8)+tlog(1.0264)=log(41.3)+tlog(1.0224)
Moving all the terms involving t to one side of the equation and the rest of the terms to the other side,
tlog(1.0264)−tlog(1.0224)=log(41.3)−log(38.8)
Factoring out the t on the left,
t(log(1.0264)−log(1.0224))=log(41.3)−log(38.8)
Dividing to solve for t
t=log(41.3)−log(38.8)log(1.0264)−log(1.0224)≈15.991 years until the populations will be equal.
Example 5.3.1b
Solve the problem above by rewriting before taking the log.
Solution
Starting at the equation
38.8(1.0264)t=41.3(1.0224)t
Divide to move the exponential terms to one side of the equation and the constants to the other side
1.0264t1.0224t=41.338.8
Using exponent rules to group on the left,
(1.02641.0224)t=41.338.8
Taking the log of both sides
log((1.02641.0224)t)=log(41.338.8)
Utilizing the exponent property on the left,
tlog(1.02641.0224)=log(41.338.8)
Dividing gives
t=log(41.338.8)log(1.02641.0224)≈15.991 years
While the answer does not immediately appear identical to that produced using the previous method, note that by using the difference property of logs, the answer could be rewritten:
t=log(41.338.8)log(1.02641.0224)=log(41.3)−log(38.8)log(1.0264)−log(1.0224)
While both methods work equally well, it often requires fewer steps to utilize algebra before taking logs, rather than relying solely on log properties.
Exercise 5.3.1
Tank A contains 10 liters of water, and 35% of the water evaporates each week. Tank B contains 30 liters of water, and 50% of the water evaporates each week. In how many weeks will the tanks contain the same amount of water?
- Answer
-
4.1874 weeks
Radioactive Decay
In an earlier section, we discussed radioactive decay – the idea that radioactive isotopes change over time. One of the common terms associated with radioactive decay is half-life.
Definition: Half Life
The half-life of a radioactive isotope is the time it takes for half the substance to decay.
Given the basic exponential growth/decay equation h(t)=abt, half-life can be found by solving for when half the original amount remains; by solving 12a=a(b)t, or more simply 12=bt. Notice how the initial amount is irrelevant when solving for half-life.
Example 5.3.2
Bismuth-210 is an isotope that decays by about 13% each day. What is the half-life of Bismuth-210?
Solution
We were not given a starting quantity, so we could either make up a value or use an unknown constant to represent the starting amount. To show that starting quantity does not affect the result, let us denote the initial quantity by the constant a. Then the decay of Bismuth-210 can be described by the equation Q(d)=a(0.87)d.
To find the half-life, we want to determine when the remaining quantity is half the original: 12a. Solving,
12a=a(0.87)d
12=0.87d
log(12)=log(0.87d)
log(12)=dlog(0.87)
d=log(12)log(0.87)≈4.977 days
This tells us that the half-life of Bismuth-210 is approximately 5 days.
Example 5.3.3
Cesium-137 has a half-life of about 30 years. If you begin with 200 mg of cesium-137, how much will remain after 30 years? 60 years? 90 years?
Solution
Since the half-life is 30 years, after 30 years, half the original amount, 100 mg, will remain.
After 60 years, another 30 years have passed, so during that second 30 years, another half of the substance will decay, leaving 50 mg.
After 90 years, another 30 years have passed, so another half of the substance will decay, leaving 25 mg.
Example 5.3.4
Carbon-14 is a radioactive isotope that is present in organic materials, and is commonly used for dating historical artifacts. Carbon-14 has a half-life of 5730 years. If a bone fragment is found that contains 20% of its original carbon-14, how old is the bone?
Solution
To find how old the bone is, we first will need to find an equation for the decay of the carbon-14. We could either use a continuous or annual decay formula, but opt to use the continuous decay formula since it is more common in scientific texts. The half life tells us that after 5730 years, half the original substance remains. Solving for the rate,
12a=aer5730
Dividing by a
12=er5730
Taking the natural log of both sides
ln(12)=ln(er5730)
Use the inverse property of logs on the right side
ln(12)=5730r
Divide by 5730
r=ln(12)5730≈−0.000121
Now we know the decay will follow the equation Q(t)=ae−0.000121t. To find how old the bone fragment is that contains 20% of the original amount, we solve for t so that Q(t)=0.20a.
0.20a=ae−0.000121t0.20=e−0.000121tln(0.20)=ln(e−0.000121t)ln(0.20)=−0.000121tt=ln(0.20)−0.000121≈13301 years
The bone fragment is about 13,300 years old.
Exercise 5.3.2
In Example 3, we learned that Cesium-137 has a half-life of about 30 years. If you begin with 200mg of Cesium-137, how long will it take until only 1 milligram remains?
- Answer
-
229.3157 , or approximately 229 years
Doubling Time
For decaying quantities, we asked how long it takes for half the substance to decay. For growing quantities we might ask how long it takes for the quantity to double.
Definition: Doubling Time
The doubling time of a growing quantity is the time it takes for the quantity to double.
Given the basic exponential growth equation h(t)=abt, doubling time can be found by solving for when the original quantity has doubled; by solving 2a=a(b)x, or more simply 2=bx. Like with decay, the initial amount is irrelevant when solving for doubling time.
Example 5.3.5
If you invest money at 8% compounded quarterly, how long will it take your money to double?
Solution
Using the compound interest equation, we can write
A(t)=P(1+0.084)4t=P(1.02)4t.
To find the doubling time, we look for the time until we have twice the original amount, so when A(t) = 2P. Notice we don’t need to know how much was invested.
2P=P(1.02)4t2=(1.02)4tlog(2)=log(1.024t)log(2)=4tlog(1.02)t=log(2)4log(1.02)≈8.751 years.
It will take about 8.75 years for the investment double in value.
Example 5.3.6
Use of a new social networking website has been growing exponentially, with the number of new members doubling every 5 months. If the site currently has 120,000 users and this trend continues, how many users will the site have in 1 year?
Solution
We can use the doubling time to find a function that models the number of site users, and then use that equation to answer the question. While we could use an arbitrary a as we have before for the initial amount, in this case, we know the initial amount was 120,000.
If we use a continuous growth equation, it would look like N(t)=120ert, measured in thousands of users after t months. Based on the doubling time, there would be 240 thousand users after 5 months. This allows us to solve for the continuous growth rate:
240=120er52=er5ln2=5rr=ln25≈0.1386
Now that we have an equation, N(t)=120e0.1386t, we can predict the number of users after 12 months:
N(12)=120e0.1386(12)=633.140 thousand users
So after 1 year, we would expect the site to have around 633,140 users.
Exercise 5.3.3
If tuition at a college is increasing by 6.6% each year, how many years will it take for tuition to double?
- Answer
-
Solving a(1+0.066)t=2a, it will take t=log(2)log(1.066)≈10.845 years, or approximately 11 years, for tuition to double.
Logarithmic Scales
For quantities that vary greatly in magnitude, a standard scale of measurement is not always effective, and utilizing logarithms can make the values more manageable. For example, if the average distances from the sun to the major bodies in our solar system are listed, you see they vary greatly.
| Planet | Distance (millions of km) |
| Mercury | 58 |
| Venus | 108 |
| Earth | 150 |
| Mars | 228 |
| Jupiter | 779 |
| Saturn | 1430 |
| Uranus | 2880 |
| Neptune | 4500 |
Placed on a linear scale – one with equally spaced values – these values get bunched up.

0 500 1000 1500 2000 2500 3000 3500 4000 4500
However, computing the logarithm of each value and plotting these new values on a number line results in a more manageable graph, and makes the relative distances more apparent.(It is interesting to note the large gap between Mars and Jupiter on the log number line. The asteroid belt is located there, which scientists believe is a planet that never formed because of the effects of the gravity of Jupiter.)
| Planet | Distance (millions of km) | log(distance) |
| Mercury | 58 | 1.76 |
| Venus | 108 | 2.03 |
| Earth | 150 | 2.18 |
| Mars | 228 | 2.36 |
| Jupiter | 779 | 2.89 |
| Saturn | 1430 | 3.16 |
| Uranus | 2880 | 3.46 |
| Neptune | 4500 | 3.65 |

Sometimes, as shown above, the scale on a logarithmic number line will show the log values, but more commonly the original values are listed as powers of 10, as shown below.

Example 5.3.7
Estimate the value of point P on the log scale above
The point P appears to be half way between -2 and -1 in log value, so if V is the value of this point,
log(V)≈−1.5
V≈10−1.5=0.0316
Example 5.3.8
Place the number 6000 on a logarithmic scale.
Solution
Since log(6000)≈3.8, this point would belong on the log scale about here:

Exercise 5.3.5
Plot the data in the table below on a logarithmic scale (From http://www.epd.gov.hk/epd/noise_educ...1/intro_5.html, retrieved Oct 2, 2010).
| Source of Sound/Noise | Approximate Sound Pressure in μ Pa (micro Pascals) |
| Launching of the Space Shuttle | 2000,000,000 |
| Full Symphony Orchestra | 2000,000 |
| Diesel Freight Train at High Speed at 25 m | 200,000 |
| Normal Conversation | 20,000 |
| Soft Whispering at 2 m in Library | 2,000 |
| Unoccupied Broadcast Studio | 200 |
| Softest Sound a human can hear | 20 |
- Answer
-

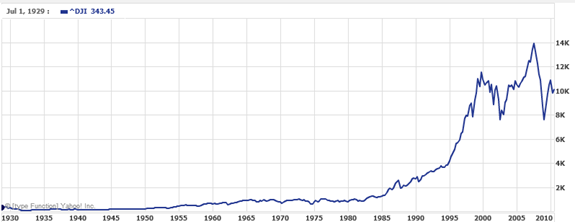
It is also common to see two dimensional graphs with one or both axes using a logarithmic scale. One common use of a logarithmic scale on the vertical axis is to graph quantities that are changing exponentially, since it helps reveal relative differences. This is commonly used in stock charts, since values historically have grown exponentially over time. Both stock charts below show the Dow Jones Industrial Average, from 1928 to 2010.
Linear scale:
Logarithmic scale:
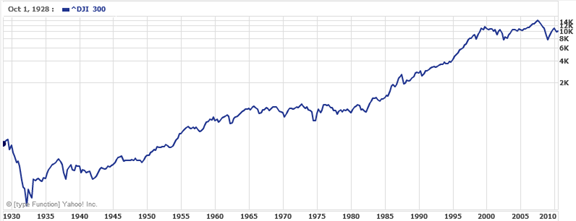
Both charts have a linear horizontal scale, but the first graph has a linear vertical scale, while the second has a logarithmic vertical scale. The first scale is the one we are more familiar with, and shows what appears to be a strong exponential trend, at least up until the year 2000.
Example 5.3.9
There were stock market drops in 1929 and 2008. Which was larger?
Solution
In the first graph, the stock market drop around 2008 looks very large, and in terms of dollar values, it was indeed a large drop. However the second graph shows relative changes, and the drop in 2009 seems less major on this graph, and in fact the drop starting in 1929 was, percentage-wise, much more significant.
Specifically, in 2008, the Dow value dropped from about 14,000 to 8,000, a drop of 6,000. This is obviously a large value drop, and amounts to about a 43% drop. In 1929, the Dow value dropped from a high of around 380 to a low of 42 by July of 1932. While value-wise this drop of 338 is much smaller than the 2008 drop, it corresponds to a 89% drop, a much larger relative drop than in 2008. The logarithmic scale shows these relative changes.
Important Topics of this Section
- Radioactive decay
- Half life
- Doubling time
- Logarithmic Scales


