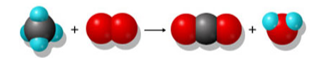
3.1: Homework Chapter 3
- Page ID
- 25739
General Questions
1. What is matter composed of at the submicroscopic level?
2. How are atoms spaced in solid matter?
3. How are atoms or molecules arranged in crystalline matter?
4. How are atoms or molecules arranged in amorphous matter?
5. You perform an experiment in which one substance is changed into a new substance. Is this a physical or chemical change?
6. What is the difference between a mixture and a pure substance?
7. In physical and chemical changes, is it possible for the amount of mass to change? Why or why not?
8. What are three methods that utilize physical changes to separate mixtures?
9. What is temperature?
10. What determines the physical properties of all matter?
11. What are the two possible classifications of pure substances?
Classifying Matter
12. Identify the following as heterogeneous or homogenous mixtures.
a. Coffee (without milk or sugar)
b. Soil
c. Chicken noodle soup
d. Blood
13. Classify each as an element or a compound.
a. Lead
b. Carbon dioxide
c. Neon
d. Pure water
14. Define the following (in your own words), and give 3 examples for each:
a. Element
b. Compound
c. Mixture
d. Pure Substance
15. How do atoms or molecules move in each:
a. Solid:
b. Liquid:
c. Gas:
16. Determine if the shape and volume are definite or indefinite for each phase:
a. Solid:
b. Liquid:
c. Gas:
17. Determine if each is a pure substance or a mixture. For pure substances, determine if it is an element or a compound. For mixtures, determine if it is homogenous or heterogeneous.
a. Salt water
b. Chocolate chip cookies
c. Helium
d. Potassium hydroxide
18. Identify which is a solid.
a. Hydrogen
b. Carbon
c. Oxygen
d. Mercury
e. Water
f. Air
19. Of the three states of matter, which is compressible?
20. Classify each pure substance as an element or a compound.
a. Titanium
b. Germanium
c. Nitrogen dioxide
d. Sodium chloride
Physical and Chemical Properties and Physical and Chemical Changes
21. Determine if each change is physical or chemical.
a. baking bread in the oven
b. stretching a piece of rubber
c. boiling water on a stove
d. grilling a steak
22. Determine if each property is physical or chemical:
a. the boiling point of water
b. the scent of flowers
c. the temperature at which dry ice sublimates
d. the flammability of gasoline
23. Determine if each of the following are an example of a physical change, a chemical change, a physical property or a chemical property:
a. iron nails rusting
b. metallic sodium exploding when exposed to water
c. the ability of dry ice to sublime
d. the shiny yellow color of gold
e. the ability of hydrogen to react with oxygen
f. ice melting
24. Which of the following is the chemical property of gasoline?
a. Boiling point
b. Density
c. Odor
d. Flammability
25. What is the difference between physical properties and chemical properties? Give an example of each.
26. Which of the following is a chemical change of water?
a. Boiling
b. Freezing
c. Electrolysis (separating into hydrogen and oxygen)
27. Iron rusts when exposed to oxygen (a process called oxidation). Is this a chemical or physical property of iron?
28. Water in a glass has a definite volume. Is this a chemical or physical property of water?
29. Water is boiled on the stove. Is it undergoing a chemical or physical change?
30. Sugar is dissolved into water. Is this a chemical or physical change?
31. Food is metabolized by the body. Is this a chemical or physical change?
32. Plants turn carbon dioxide and water into glucose. Is this a chemical or physical change?
Conservation of Mass
33. In your own words, define the law of conservation of mass.
34. In a chemical reaction, 20.0 g of reactant A reacts with 3.00 g of reactant B. How many grams of product are produced?
35. In the reaction of 2.4 g of carbon and 4.9 g of oxygen, how many grams of carbon dioxide are produced?
36. Which of the following is accurate in terms of the law of conservation of mass? (Assume the products discussed are the only products of the reactions).
a. The reaction of 5.0 g of hydrogen with 2.3 g of oxygen produces 8.5 g of water vapor.
b. The reaction of 9.8 g of nitrogen with 2.3 g of oxygen produces 12.1 g of nitrogen dioxide
37. In electrolysis, water is separated chemically into hydrogen and oxygen. How much water is required to produce 56.8 g of hydrogen and 450.8 g of oxygen?
38. In the reaction of 89.4 g of sodium with 34.7 g of chlorine, how many grams of sodium chloride are produced?
Converting Between Temperature Scales
39. Convert 45° F to Celsius
40. Convert 106 K to Fahrenheit
41. Convert -78° C to Kelvin
42. Convert 0° F to Kelvin and Celsius
43. Convert 100° C to Fahrenheit
44. Convert 400 K to Celsius
Challenge Problems
45. The boiling point of water at sea level is 100° C. What temperature is this in Kelvin? In Fahrenheit?
46. At what temperature does the Fahrenheit scale equal the Celsius scale?
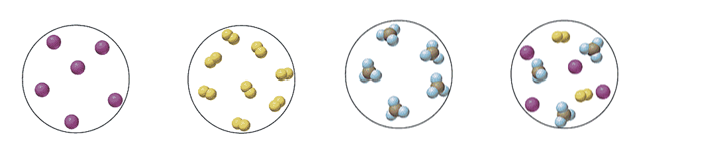
47. Label each of the following as a mixture, a compound, an atomic element or a molecular element.
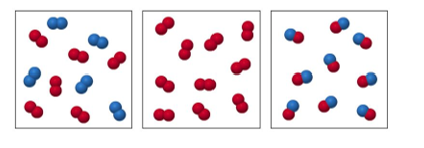
48. Label each as a pure substance or a mixture.
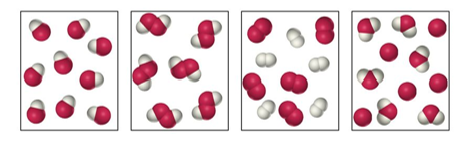
49. Label each as a pure substance or a mixture.
50. Does the following represent a chemical change or a physical change?