1.25: Converting Units of Volume
- Page ID
- 153129
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)You may use a calculator throughout this module.

Just as we saw with area, converting between units of volume requires us to be careful because cubic units behave differently than linear units.
Quantities of mulch, dirt, or gravel are often measured by the cubic yard. How many cubic feet are in one cubic yard?
\(1\) yard = \(3\) feet, so we can divide the length into three sections, the width into three sections, and the height into three sections to convert all three dimensions of the cube from yards to feet. This forms a \(3\) by \(3\) by \(3\) cube, which shows us that \(1\) cubic yard equals \(27\) cubic feet. The linear conversion ratio of \(1\) to \(3\) means that that the conversion ratio for the volumes is \(1\) to \(3^3\), or \(1\) to \(27\).
Here’s another way to think about it without a diagram: \(1\text{ yd}=3\text{ ft}\), so \((1\text{ yd})^3=(3\text{ ft})^3\). To remove the parentheses, we must cube the number and cube the units: \((3\text{ ft})^3=3^3\text{ ft}^3=27\text{ ft}^3\).
More generally, we need to cube the linear conversion factors when converting units of volume. If the linear units have a ratio of \(1\) to \(n\), the cubic units will have a ratio of \(1\) to \(n^3\).
U.S. System: Converting Measurements of Volume
\(1\text{ ft}^3=1,728\text{ in}^3\)
\(1\text{ yd}^3=27\text{ ft}^3\)
\(1\text{ yd}^3=46,656\text{ in}^3\)
- True story: A friend at the National Guard base gave us three long wooden crates to use as raised planting beds. (The crates probably carried some kind of weapons or ammunition, but our friend wouldn’t say.) Henry, who was taking geometry in high school, was asked to measure the crates and figure out how much soil we needed. The inside dimensions of each crate were \(112\) inches long, \(14\) inches wide, and \(14\) inches deep. We wanted to fill them most of the way full with soil, leaving about \(4\) inches empty at the top. How many cubic yards of soil did we need to order from the supplier?
- True story, continued: We decided to check our answer and did a rough estimate by rounding each dimension to the nearest foot, then figuring out the volume from there. Did this give the same result?

We can convert between units of volume and liquid capacity. As you might expect, the numbers are messy in the U.S. system.
\(1\text{ fl oz}\approx1.8047\text{ in}^3\)
\(1\text{ ft}^3\approx7.4805\text{ gal}\)
- A circular wading pool has a diameter of roughly 5 feet and a depth of 6 inches. How many gallons of water are required to fill it about \(80\%\) of the way full?
- A standard U.S. soda pop can has a diameter of \(2\dfrac{1}{2}\) inches and a height of \(4\dfrac{3}{4}\) inches. Verify that the can is able to hold \(12\) fluid ounces of liquid.
- A water jug that is roughly cube-shaped is advertised with dimensions \(10.51\) inches deep, \(10.51\) inches wide, and \(10.51\) inches high. How many gallons is its capacity?

Metric System: Converting Measurements of Volume
\(1\text{ cm}^3=1\text{ cc}=1\text{ mL}\)
\(1\text{ cm}^3=1,000\text{ mm}^3\)
\(1\text{ m}^3=1,000,000\text{ cm}^3\)
\(1\text{ L}=1,000\text{ cm}^3\)
\(1\text{ m}^3=1,000\text{ L}\)
It’s no surprise that the metric conversion ratios for volume are all powers of \(10\)… but they are actually powers of \(1,000\), or \(10^3\), because the linear conversions get cubed when we go three-dimensional.
- The circular soaking tubs at Hot Lake Springs in La Grande, Oregon have an inner diameter of \(2.00\) meters, and the water has a depth of \(0.75\) meters. What is the approximate volume of water, in liters, in one of the tubs?

- A \(1.5\text{ L}\) carton of orange juice has a square base \(9.5\) centimeters on each side, and the height of the rectangular section is \(19\) centimeters. (Ignore the triangular section at the top.) Verify that the carton holds \(1.5\) liters of juice.
- A \(1.89\text{ L}\) carton of POG juice has a square base \(9.5\) centimeters on each side, and the height of the rectangular section is \(19.5\) centimeters. This is not enough volume to hold \(1.89\) liters of juice unless the sides of the carton bulge out. If you unfold the triangular top section to make a rectangular column and squeeze the sides of the carton so they aren’t bulging out, what height will the juice rise to?

Both Systems: Converting Measurements of Volume
Converting volumes between the U.S. and metric systems will of course involve messy decimal values. For example, because \(1\text{ in}=2.54\text{ cm}\), we can cube both numbers and find that \(1\text{ in}^3=(2.54\text{ cm})^3=\) \(16.387064\text{ cm}^3\). The conversions in the table below are rounded to five significant figures.
- A large dumpster in Iceland has a volume of \(15\) cubic meters. Convert this to cubic yards.
- Suppose you need to know the volume of an Icelandic dumpster in cubic feet. Convert \(15\text{ m}^3\) to \(\text{ ft}^3\).

- A “two yard” dumpster at a campground in Washington has a volume of \(2\) cubic yards. Convert this to cubic meters.

- The engine of a 1964 Corvette has 8 cylinders with a bore (diameter) of \(4.00\) inches and a stroke (height) of \(3.25\) inches. Find the total displacement (volume) of the cylinders in this engine, rounded to the nearest cubic inch.[1]
- Convert the displacement of the Corvette engine into cubic centimeters.
- Convert the displacement of the Corvette engine into liters.

- A hardware store sells pea gravel in \(0.5\text{ ft}^3\) bags. The bags are also marked \(14\text{ L}\). Verify that these two measures are equivalent.
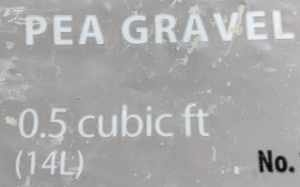
Density
The density of a material is its weight per volume such as pounds per cubic foot, or mass per volume such as grams per cubic centimeter. Multiplying the volume of an object by its density will give its weight or mass.
- A \(0.5\text{ ft}^3\) bag of pea gravel weighs approximately 50 pounds. Determine the density of pea gravel in pounds per cubic foot.
- The standard size of a gold bar in the U.S. Federal Reserve is \(7\) inches by \(3\dfrac{5}{8}\) inches by \(1\dfrac{3}{4}\) inches.[2] The density of gold is \(0.698\) pounds per cubic inch. How much does one gold bar weigh?
- A cylindrical iron bar has a diameter of \(3.0\) centimeters and a length of \(20.0\) centimeters. The density of iron is \(7.87\) grams per cubic centimeter. What is the bar’s mass, in kilograms?
Volumes of Similar Solids
Earlier in this module, it was stated that if the linear units have a ratio of \(1\) to \(n\), the cubic units will have a ratio of \(1\) to \(n^3\). This applies to similar solids as well.
If the linear dimensions of two similar solids have a ratio of \(1\) to \(n\), then the volumes will have a ratio of \(1\) to \(n^3\).
We’ll verify this in the following exercises.

A table tennis (ping pong) ball has a diameter of \(4\) centimeters. A wiffle® ball has a diameter twice that of a table tennis ball.
- Determine the volume of the wiffle® ball.
- Determine the volume of the table tennis ball.
- What is the ratio of the volumes of the two balls?
Rectangular solid \(A\) has dimensions \(3\) inches by \(4\) inches by \(5\) inches. Rectangular solid \(B\) has dimensions triple those of \(A\)’s.
- Determine the volume of the larger solid, \(B\).
- Determine the volume of the smaller solid, \(A\).
- What is the ratio of the volumes of the two solids?
- The formula is often written as \(V=\dfrac{\pi}{4}\cdot{b^2}\cdot{s}\cdot{c}\), where \(c\) is the number of cylinders. ↵
- Source: https://www.usmint.gov/about/mint-tours-facilities/fort-knox↵

