7.2: Scientific Notation
- Page ID
- 19892
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We begin this section by examining powers of ten.
\[\begin{align*} 10^1 &= 10\\ 10^2 &= 10\cdot 10 = 100\\ 10^3 &= 10\cdot 10\cdot 10 = 1,000\\ 10^4 &= 10\cdot 10\cdot 10\cdot 10 = 10,000 \end{align*} \nonumber \]
Note that the answer for \(10^3\) is a one followed by three zeros. The answer for \(10^4\) is a one followed by four zeros. Do you see the pattern?
Nonnegative powers of ten
In the expression \(10^n\), the exponent matches the number of zeros in the answer. Hence, \(10^n\) will be a \(1\) followed by \(n\) zeros.
Example \(\PageIndex{1}\)
Simplify: \(10^9\).
Solution
\(10^9\) should be a \(1\) followed by \(9\) zeros.\[10^9 =1 ,000,000,000 \nonumber \]
Exercise \(\PageIndex{1}\)
Simplify: \(10^6\).
- Answer
-
\(1,000,000\)
Next, let’s examine negative powers of ten.
\[\begin{align*} 10^{-1} &= \dfrac{1}{10} = 0.1\\ 10^{-2} &= \dfrac{1}{100} = 0.01\\ 10^{-3} &= \dfrac{1}{1000} = 0.001\\ 10^{-4} &= \dfrac{1}{10000} = 0.0001 \end{align*} \nonumber \]
Note that the answer for \(10^{−3}\) has three decimal places and the answer for \(10^{−4}\) contains four decimal places.
Negative powers of ten
In the expression \(10^{−n}\), the exponent matches the number of decimal places in the answer. Hence, \(10^{−n}\) will have n decimal places, the first \(n−1\) of which are zeros and the digit in the nth decimal place is a \(1\).
Example \(\PageIndex{2}\)
Simplify: \(10^{−7}\).
Solution
\(10^{−7}\) should have seven decimal places, the first six of which are zeros, and the digit in the seventh decimal place is a \(1\).\[10^{−7} = 0 .0000001 \nonumber \]
Exercise \(\PageIndex{2}\)
Simplify: \(10^{−5}\).
- Answer
-
\(0.00001\)
Multiplying Decimal Numbers by Powers of Ten
Let’s multiply \(1.234567\) by \(10^3\), or equivalently, by \(1,000\).
\[\begin{array}{ccc} 1.234567 \\ \;\;\times 1000\\ \hline 1234.567000 \end{array} \nonumber \]
The sum total of digits to the right of the decimal point in \(1.234567\) and \(1000\) is \(6\). Therefore, we place the decimal point in the product so that there are six digits to the right of the decimal point.
However, the trailing zeros may be removed without changing the value of the product. That is, \(1.234567\) times \(1000\) is \(1234.567\). Note that the decimal point in the product is three places further to the right than in the original factor. This observation leads to the following result.
Multiplying by a nonnegative power of ten
Multiplying a decimal number by \(10^n\), where \(n = 0, 1, 2, 3, \ldots ,\) will move the decimal point \(n\) places to the right.
Example \(\PageIndex{3}\)
Simplify: \(325.6783×10^2\).
Solution
Multiplying by \(10^2\) will move the decimal point two places to the right. Thus:\[325.6783×10^2 = 32,567.83 \nonumber \]
Exercise \(\PageIndex{3}\)
Simplify: \(23.57889×10^3\)
- Answer
-
\(23,578.89\)
Example \(\PageIndex{4}\)
Simplify: \(1.25×10^5\).
Solution
Multiplying by \(10^5\) will move the decimal point two places to the right. In this case, we need to add zeros at the end of the number to accomplish moving the decimal \(5\) places to the right.\[1.25×105 = 125,000 \nonumber \]
Exercise \(\PageIndex{14}\)
Simplify: \(2.35×10^4\)
- Answer
-
\(23,500\)
Let’s multiply \(453.9\) by \(10^{−2}\), or equivalently, by \(0.01\).
\[\begin{array}{ccc} 453.9 \\ \times 0.01\\ \hline 4.539 \end{array} \nonumber \]
The sum total of digits to the right of the decimal point in \(453.9\) and \(0.01\) is \(3\). Therefore, we place the decimal point in the product so that there are \(3\) digits to the right of the decimal point. That is, \(453.9×10^{−2} = 4.539\). Note that the decimal point in the product is two places further to the left than in the original factor. This observation leads to the following result.
Multiplying by a negative power of ten
Multiplying a decimal number by \(10^{−n}\), where \(n = 1, 2, 3, \ldots ,\) will move the decimal point \(n\) places to the left.
Example \(\PageIndex{5}\)
Simplify: \(14,567.8×10^{−3}\).
Solution
Multiplying by \(10^{−3}\) will move the decimal point three places to the left. Thus: \[14,567.8×10^{−3} = 14 .5678 \nonumber \]
Exercise \(\PageIndex{5}\)
Simplify: \(3,854.2×10^{−1}\)
- Answer
-
\(385 .42\)
Example \(\PageIndex{6}\)
Simplify: \(4.3×10^{−4}\).
Solution
Multiplying by \(10^{−4}\) will move the decimal point four places to the left. In this case, we need to add some leading zeros at the beginning of the number to accomplish moving the decimal \(4\) places to the left.\[4.3×10^{−4} =0.00043\nonumber \]. Note also the leading zero before the decimal point. Although \(.00043\) is an equivalent number, the form \(0.00043\) is preferred in mathematics and science.
Exercise \(\PageIndex{6}\)
Simplify: \(2.2×10^{−2}\)
- Answer
-
\(0.022\)
Scientific Notation Form
We start by defining the form of a number called scientific notation.
Scientific notation
A number having the form\[a×10^b \nonumber \]where \(b\) is an integer and \(1 ≤| a| < 10\), is said to be in scientific notation.
The requirement \(1 ≤| a| < 10\) says that the magnitude of a must be a least \(1\) and less than \(10\).
- The number \(12.34×10^{−4}\) is not in scientific notation because \(|12.34| = 12.34\) is larger than \(10\).
- The number \(−0.95×10^3\) is not in scientific notation because \(|−0.95|= 0.95\) is less than \(1\).
- The number \(7.58×10^{−12}\) is in scientific notation because \(|7.58|=7.58\) is greater than or equal to \(1\) and less than \(10\).
- The number \(−1.0×10^{15}\) is in scientific notation because \(|−1.0|=1.0\) is greater than or equal to \(1\) and less than \(10\).
After contemplating these examples, it follows that a number in scientific notation should have exactly one of the digits \(1, 2, 3, \ldots , 9\) before the decimal point. Exactly one, no more, no less. Thus, each of the following numbers is in scientific notation.
\[4.7×10^8, \quad −3.764×10^{−1}, \quad 3.2×10^0, \quad \text {and} \quad −1.25×10^{−22} \nonumber \]
Placing a Number in Scientific Notation
To place a number into scientific notation, we need to move the decimal point so that exactly one of the digits \(1, 2, 3, \ldots , 9\) remains to the left of the decimal point, then multiply by the appropriate power of \(10\) so that the result is equivalent to the original number.
Example \(\PageIndex{7}\)
Place the number \(1,234\) in scientific notation.
Solution
Move the decimal point three places to the left so that it is positioned just after the \(1\). To make this new number equal to \(1,234\), multiply by \(10^3\). Thus:\[1,234 = 1.234×10^3 \nonumber \]
Check: Multiplying by \(10^3\) moves the decimal three places to the right, so:\[1.234×10^3 =1,234 \nonumber \] This is the original number, so our scientific notation form is correct.
Exercise \(\PageIndex{7}\)
Place the number \(54,321\) in scientific notation.
- Answer
-
\(5 .4321×10^4\)
Example \(\PageIndex{8}\)
Place the number \(0.000025\) in scientific notation.
Solution
Move the decimal point five places to the right so that it is positioned just after the \(2\). To make this new number equal to \(0.000025\), multiply by \(10^{−5}\). Thus: \[0.000025 = 2.5×10^{−5} \nonumber \]
Check: Multiplying by \(10^{−5}\) moves the decimal five places to the left, so:\[2.5×10^{−5} = 0 .000025 \nonumber \]This is the original number, so our scientific notation form is correct.
Exercise \(\PageIndex{8}\)
Place the number \(0.0175\) in scientific notation.
- Answer
-
\(1.75×10^{−2}\)
Example \(\PageIndex{9}\)
Place the number \(34.5×10^{−11}\) in scientific notation.
Solution
First, move the decimal point one place to the left so that it is positioned just after the three. To make this new form equal to \(34.5\), multiply by \(10^1\).\[34.5×10^{−11} = 3.45×10^1×10^{−11} \nonumber \]Now, repeat the base \(10\) and add the exponents.
\[=3 .45×10^{−10} \nonumber \]
Exercise \(\PageIndex{9}\)
Place the number \(756.98×10^{−5}\) in scientific notation.
- Answer
-
\(7.5698×10^{−3}\)
Example \(\PageIndex{10}\)
Place the number \(0.00093×10^{12}\) in scientific notation.
Solution
First, move the decimal point four places to the right so that it is positioned just after the nine. To make this new form equal to \(0.00093\), multiply by \(10^{−4}\). \[0.00093×10^{12} = 9.3×10^{−4} ×10^{12} \nonumber \]
Now, repeat the base \(10\) and add the exponents.\[=9.3×10^8 \nonumber \]
Exercise \(\PageIndex{10}\)
Place the number \(0.00824×10^8\) in scientific notation.
- Answer
-
\(8.24×10^5\)
Scientific Notation and the Graphing Calculator
The TI-84 graphing calculator has a special button for entering numbers in scientific notation. Locate the “comma” key just about the number \(7\) key on the calculator’s keyboard (see Figure \(\PageIndex{1}\)). Just above the “comma” key, printed on the calculator’s case is the symbol EE. It’s in the same color as the 2nd key, so you’ll have to use the 2nd key to access this symbol.

We know that \(2.3 × 10^2 = 230\). Let’s see if the calculator gives the same interpretation.
- Enter \(2.3\).
- Press the 2nd key, then the comma key. This will put E on the calculator view screen.
- Enter a \(2\).
- Press ENTER.

The result of these steps is shown in the first image in Figure \(\PageIndex{2}\). Note that the calculator interprets \(2.3\mathbf{E}2\) as \(2.3×10^2\) and gives the correct answer, \(230\). You can continue entering numbers in scientific notation (see the middle image in Figure \(\PageIndex{2}\)). However, at some point the numbers become too large and the calculator responds by outputting the numbers in scientific notaiton. You can also force your calculator to display numbers in scientific notation in all situations, by first pressing the MODE key, then selecting SCI on the first line and pressing the ENTER key (see the third image in Figure \(\PageIndex{2}\)). You can return your calculator to “normal” mode by selecting NORMAL and pressing the ENTER key.
Example \(\PageIndex{11}\)
Use the graphing calculator to simplify: \[(2.35×10^{−12})(3.25×10^{−4}) \nonumber \]
Solution
First, note that we can approximate \((2.35×10^{−12})(3.25×10^{−4})\) by taking the product of \(2\) and \(3\) and adding the powers of ten.
\[\begin{align*} &(2.35\times 10^{-12})(3.25\times 10^{-4}) \\ &\approx (2\times 10^{-12})(3\times 10^{-4}) \quad \color {Red} \text {Approximate: } 2.35\approx 2 \text { and } 3.25\approx 3 \\ &\approx 6\times 10^{-16} \quad \color {Red} 2\cdot 3 = 6 \text { and } 10^{-12}\cdot 10^{-4} = 10^{-16} \end{align*} \nonumber \]
The graphing calculator will provide an accurate answer. Enter \(2.35\mathbf{E}-12\), press the “times” button, then enter 3.25E-4 and press the ENTER button. Be sure to use the “negate” button and not the “subtract” button to produce the minus sign. The result is shown in Figure \(\PageIndex{3}\).

Thus, \((2.35×10^{−12})(3.25×10^{−4})=7 .6375×10^{−16}\). Note that this is fairly close to our estimate of \(6 ×10^{−16}\).
Exercise \(\PageIndex{11}\)
Use the graphing calculator to simplify: \[(3.42×10^6)(5.86×10^{−9}) \nonumber \]
- Answer
-
\(2.00412×10^{−2}\)
Reporting your answer on your homework
After computing the answer to Example \(\PageIndex{11}\) on your calculator, write the following on your homework:\[(2.35×10^{−12})(3.25×10^{−4})=7.6375×10^{−16} \nonumber \]Do not write \(7.6375\mathbf{E}-16\).
Example \(\PageIndex{12}\)
Use the graphing calculator to simplify:\[\dfrac{6.1\times 10^{-3}}{(2.7\times 10^4)(1.1\times 10^8)} \nonumber \]
Solution
Again, it is not difficult to produce an approximate answer.
\[\begin{align*} &\dfrac{6.1\times 10^{-3}}{(2.7\times 10^4)(1.1\times 10^8)} \\ &\approx \dfrac{6\times 10^{-3}}{(3\times 10^4)(1\times 10^8)} \quad \color {Red} 6.1\approx 6, 2.7\approx 3, \text { and } 1.1\approx 1 \\ &\approx \dfrac{6\times 10^{-3}}{3\times 10^{12}} \quad \color {Red} 3\cdot 1=3 \text { and } 10^{4}\cdot 10^{8} = 10^{12}\\ &\approx \dfrac{6}{3}\cdot \dfrac{10^{-3}}{10^{12}} \quad \color {Red} \dfrac{ac}{bd}=\dfrac{a}{b}\cdot \dfrac{c}{d}\\ &\approx 2\times 10^{-15} \quad \color {Red} \dfrac{6}{3}=2 \text { and } \dfrac{10^{-3}}{10^{12}}=10^{-15} \end{align*} \nonumber\]
Let’s get a precise answer with our calculator. Enter the numerator as \(6.1\mathbf{E}3\), then press the “division” button. Remember that we must surround the denominator with parentheses. So press the open parentheses key, then enter \(2.7\mathbf{E}4\). Press the “times” key, then enter \(1.1\mathbf{E}8\). Press the close parentheses key and press the ENTER button. The result is shown in Figure \(\PageIndex{4}\).

Thus, \(6.1×10^{−3}/(2.7×10^4 ×1.1×10^8)=2 .05387205×10^{−15}\). Note that this is fairly close to our estimate of \(2×10^{−15}\).
Exercise \(\PageIndex{12}\)
Use the graphing calculator to simplify: \[\dfrac{2.6\times 10^{4}}{(7.1\times 10^{-2})(6.3\times 10^7)} \nonumber \]
- Answer
-
\(5.8126537×10^{−3}\)
Example \(\PageIndex{13}\)
Isaac Newton’s universal law of gravitation is defined by the formula \[F = \dfrac{GmM}{r^2} \nonumber \] where \(F\) is the force of attraction between two objects having mass \(m\) and \(M\), \(r\) is the distance between the two objects, and \(G\) is Newton’s gravitational constant defined by:\[G =6 .67428\times 10^{-11} \text {N(m/kg)}^2 \nonumber\]Given that the mass of the moon is \(7.3477×10^{22}\) kilograms (kg), the mass of the earth is \(5.9736×10^{24}\) kilograms (kg), and the average distance between the moon and the earth is \(3.84403×10^8\) meters (m), find the force of attraction between the earth and the moon (in newtons (N)).
Solution
Plug the given numbers into Newton’s universal law of gravitation.
\[F=\dfrac{GmM}{r^2} \nonumber \]
\[F=\dfrac{(6.673\times 10^{-11})(7.3477\times 10^{22})(5.9736\times 10^{24})}{(3.84403\times 10^8)^2} \nonumber \]
Enter the expression into your calculator (see Figure \(\PageIndex{5}\)) as:
\[(6.673\mathbf{E}-11*7.3477\mathbf{E}22*5.9736\mathbf{E}24)/(3.84403\mathbf{E}8)\wedge 2 \nonumber\]
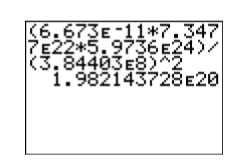
Hence, the force of attraction between the earth and the moon is approximately \(1.98×10^{20}\) newtons (N).
Exercise \(\PageIndex{13}\)
The mass of the International Space Station is \(450,000\) kg, and its average distance to the center of the earth is \(387,000\) m. Find the force of attraction between the earth and the station (in newtons (N)).
- Answer
-
\(≈ 1.20×109 N\)
Example \(\PageIndex{14}\)
The closest star to the earth is Alpha Centauri, \(4.37\) light-years from the earth. A light-year is the distance that light will travel in one-year’s time. The speed of light is \(186,000\) miles per second. How many miles from the earth is Alpha Centauri?
Solution
Because the speed of light is measured in miles per second, let’s first compute the number of seconds in \(4.37\) years. Because there are \(365\) days in a year, \(24\) hours in a day, \(60\) minutes in an hour, and \(60\) seconds in a minute, we can write:
\[\begin{aligned} 4.37 \text {yr} &= 4.37\text {yr}\times 365\dfrac{\text {day}}{\text {yr}}\times 24\dfrac{\text {hr}}{\text {day}}\times 60\dfrac{\text {min}}{\text {hr}}\times 60\dfrac{\text {s}}{\text {min}}\\ &= 4.37{\color {Red}\not {\color {Black}\text {yr}}}\times 365\dfrac{\color {Red}\not {\color {Black}\text {day}}}{\color {Red}\not {\color {Black}\text {yr}}}\times 24\dfrac{\color {Red}\not {\color {Black}\text {hr}}}{\color {Red} \not {\color {Black}\text {day}}}\times 60\dfrac{\color {Red}\not {\color {Black}\text {min}}}{\color {Red}\not {\color {Black}\text {hr}}}\times 60\dfrac{\text {s}}{\color {Red}\not {\color {Black}\text {min}}} \end {aligned} \nonumber \]
Note how the units cancel, indicating that the final answer is in seconds. With our calculator mode set to scientific notation (see the image on the right in Figure \(\PageIndex{2}\)), we multiply the numbers to get the result shown in Figure \(\PageIndex{6}\). Rounding, the number of seconds in \(4.37\) years is approximately \(1.38 × 10^8\) seconds.
Next, we compute the distance the light travels in \(4.37\) years. Using the fact that the distance traveled equals the speed times the time traveled, we have:
\[\begin{aligned} \text {Distance} &= \text {Speed}\times \text {Time}\\ &= 1.86\times 10^5\dfrac{\text {mi}}{\text {s}}\cdot 1.38\times 10^8\text {s}\\ &= 1.86\times 10^5\dfrac{\text {mi}}{\color {Red}\not {\color {Black}\text {s}}}\cdot 1.38\times 10^8 {\color {Red}\not {\color {Black}\text {s}}} \end {aligned} \nonumber\]
Note how the units cancel, indicating that our answer is in miles. Again, with our calculator set in scientific notation mode, we compute the product of \(1.86×10^5\) and \(1.38×10^8\). The result is shown in the image on the right in Figure \(\PageIndex{6}\).
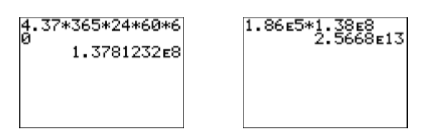
Thus, the star Alpha Centauri is approximately \(2.5668×10^{13}\) miles from the earth, or \[2.5668×10^{13} \text {miles} ≈ 25,668,000,000,000 \text {miles} \nonumber \]pronounced “twenty-five quadrillion, six hundred sixty-eight trillion miles.”
Exercise \(\PageIndex{14}\)
The star Sirius is \(8.58\) light-years from the earth. How many miles from the earth is Sirius?
- Answer
-
\(≈ 5.2425×10^{13}\) miles


